Which Best Describes How Heat Energy Moves Within a System
Which statement best describes the energy changes that occurred. Cool objects transfer heat energy to warmer objects.

Which Best Describes How Heat Energy Moves Within A System Lisbdnet Com
HURRY PLEASEWhich best describes how heat energy moves within a system.

. September 30 2016 at 615 am. In other words heat is energy while temperature is a measure of energy. The processes are known as conduction convection and radiation.
There are three ways in which heat is transferred they are. This means heat is a form of kinetic energy. So when two objects with different temperatures are placed adjacently in contact with each other then heat will transfer from hotter object to colder object.
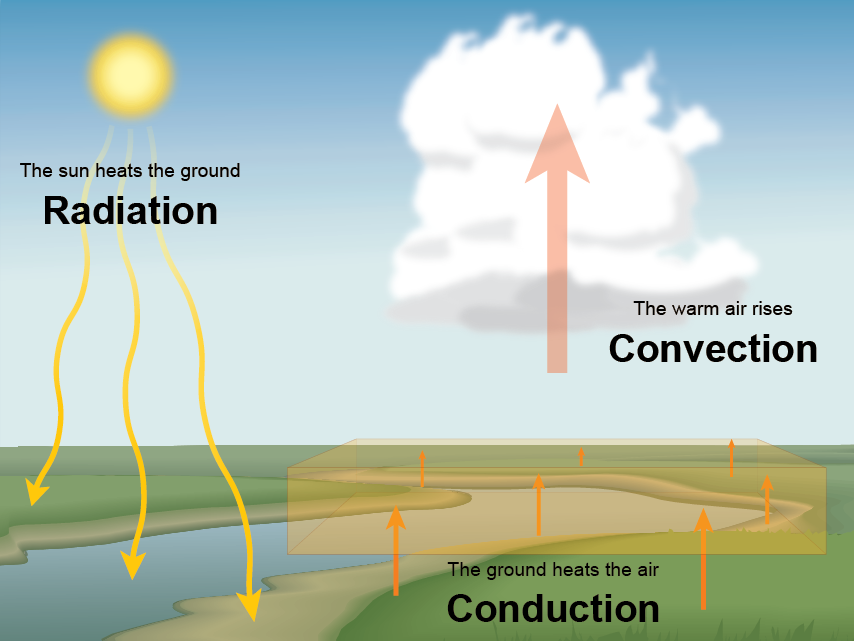
This is how the heat from the Sun gets to Earth. An object can gain heat or lose heat but it cannot have heat. The energy transferred between objects at different temperatures.
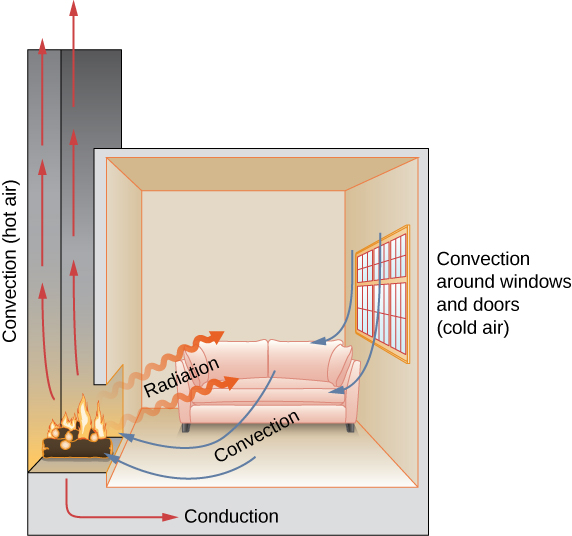
Heat refers to the transfer of energy between systems or bodies whereas temperature is determined by the energy contained within a singular system or body. If you have stood in front of a fireplace or near a campfire you have felt the heat transfer known as radiation. Heat moves naturally by any of three means.
To solve calorimetry problems. Water loses energy when it changes phase from. Heat energy is the result of the movement of tiny particles called atoms molecules or ions in solids liquids and gases.
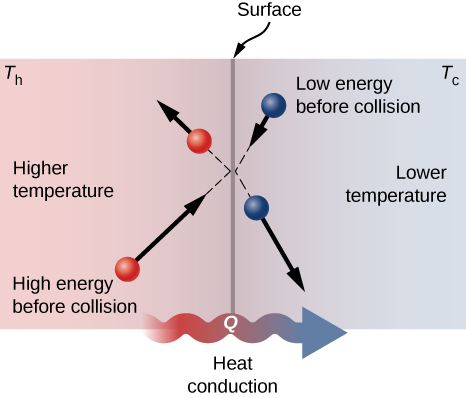
They would stop moving. For example an ice cube has heat energy and so does a glass of. Here there must be a contact between the two bodies.
Which best describes how heat energy move within a system. This is so because the particles of the hot objects have a higher kinetic energy than the cooler object. Cool objects transfer heat energy to warmer objects.
Matter may be organic or inorganic. Use the principle of the conservation of energy in an isolated system and the specific heat equation Q mcΔT. What would be the effect on the particles if more heat is supplied to the system.
Heat always moves from an object of _____ temperature to an object of _____ temperature. Hence energy flows through successive trophic levels in a food chain. As a result molecules gain kinetic energy and collide more rapidly.
4In an isolated system two copper bars at different temperatures transfer energy until both are at the. Rock A has twice the mass of rock B. The side of your body nearest the fire warms.
The transfer or flow due to the difference in temperature between the two objects is called heat. Two rocks A and B are raised to the top of a cliff. Since this energy warms the earths surface and atmosphere some of it is or becomes heat energy.
Radiation conduction and convection. Heat energy is lost to the system over time. What describes how heat energy moves within a systemConduction is one of the three main ways that heat energy moves from place to place.
Heat describes the transfer of thermal energy between molecules within a system and is measured in Joules. Throughout the universe its natural for energy to flow from one place to another. Heat energy can be transferred from one object to another.
Hence this energy is given off in the form of heat. Radiation happens when heat moves as energy waves called infrared waves directly from its source to something else. Heat is a measure of change never a property possessed by an object or system.
Which best describes how heat energy moves within a system. Which best describes how heat energy moves within a system. Heat energy is transferred from warmer objects to cooler objects.
Heat energy is lost to the system over timeC. The horizontal surface exerts a force of friction of 300 N on m 2If the system is released from rest use energy concepts to find the speed of m 3 after it moves down 400 m. Adding heat will increase a bodys temperature while removing heat will lower the temperature thus changes in.
Heat energy is transferred from warmer objects to cooler objects. Up to 256 cash back Three objects with masses m 1 500 kg m 2 100 kg and m 3 1500 kg respectively are attached by strings over frictionless pulleys. Heat moves in three ways.
Heat energy is transferred from warmer objects to cooler objects. Cool objects transfer heat energy to warmer objects. Compared to rock A rock B has.
They would speed up. Which change occurs at the point when a liquid becomes a solid. The organic and inorganic matter is produced exchanged and moved within the ecosystem and this is called cycling.
Heat energy is a form of kinetic energy because heat is related to the motion of particles. They would slow down. Cool objects transfer heat energy to warmer objects.
When we provide heat to a substance then its molecules start to vibrate more rapidly. From hot toward cold. The study of the thermal-energy transfer between different substances.
In fact all hot things radiate heat to cooler things. The other two ways heat moves around are radiation and convection. The warmth felt near a hot fire.
Heat energy is lost to the system over time. The average translational kinetic energy of the particles in an object. And unless people interfere thermal energy or heat naturally flows in one direction only.
Conduction is the process by which heat energy is transmitted through collisions between neigh. Heat energy is lost to the system over timeC. There are three ways heat is transferred into and through the atmosphere.
Therefore it is classified as a process variable. The total amount of energy possessed by the particles in an object. Heat measures how energy moves or flows.
It changes from solid to liquid and heat is released. The energy that an object has as a result of its temperature. Here heat is transferred from a hot object to a cooler object when both objects come in contact with one another.

Frost Protection Fundamentals Practice And Economics Volume 1

Methods Of Heat Transfer Boundless Physics

Heat Transfer Conduction Convection Radiation Videos And Case Study
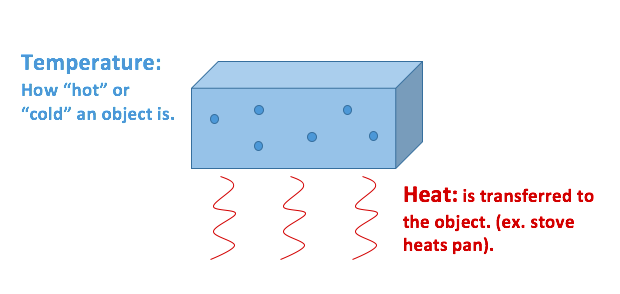
Heat Vs Temperature Energy Education
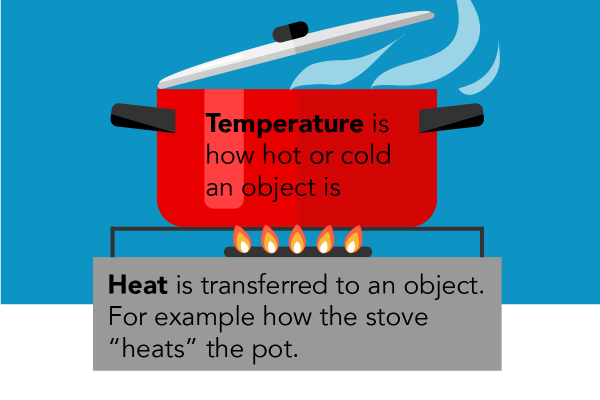
Introduction To Heat Transfer Let S Talk Science

Part A Decide Which Of The Following Statements Correctly Describes Heat If The Statement Describes Heat Correctly Write H Before The Number If Ppt Video Online Download

Heat A Simple Introduction To The Science Of Heat Energy
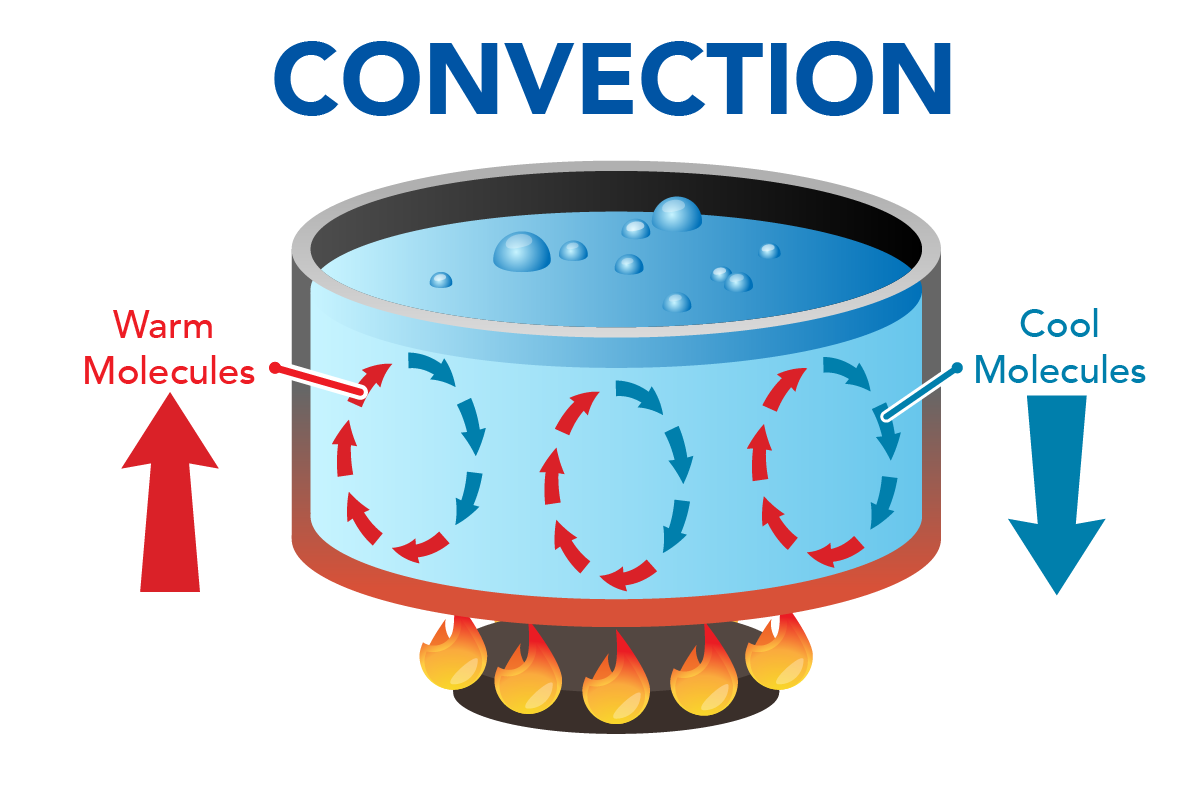
Introduction To Heat Transfer Let S Talk Science
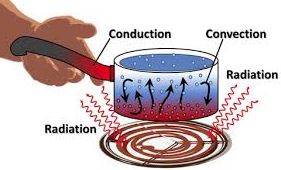
Thermal Energy 2019 Science Quiz Quizizz

Part A Decide Which Of The Following Statements Correctly Describes Heat If The Statement Describes Heat Correctly Write H Before The Number If Ppt Video Online Download
Mechanisms Of Heat Transfer University Physics Volume 2
Mechanisms Of Heat Transfer University Physics Volume 2
Nws Jetstream The Transfer Of Heat Energy



Comments
Post a Comment